Abstract
The marine alginate lyase from Streptomyces sp. ALG-5, which specifically degrades poly-G block of alginate, was functionally expressed as a His-tagged form with an Escherichia coli expression system. The recombinant alginate lyase expressed with pColdI at 15 °C exhibited the highest alginate-degrading activity. The recombinant alginate lyase was efficiently immobilized onto two types of magnetic nanoparticles, superparamagnetic iron oxide nanoparticle, and hybrid magnetic silica nanoparticle, based on the affinity between His-tag and Ni2+ that displayed on the surfaces of nanoparticles. An alginate oligosaccharide mixture consisting of dimer and trimer was prepared by the immobilized alginate lyase. The immobilized enzymes were re-used repeatedly more than 10 times after magnetic separation.
Similar content being viewed by others
Introduction
Alginate is a polysaccharide consisting of 1 → 4 linked copolymers of α-l-guluronate (G) and its C5 epimer, β-d-mannuronate (M) [1–3]. Alginate lyase is the enzyme that catalyzes the β-elimination breakage of the glycosidic bond. Alginate lyases are classified as poly-mannuronate lyase, which is specific for poly-mannuronate (poly-M) block and poly-guluronate lyase that is specific for poly-guluronate (poly-G) block [4–6]. Alginate oligosaccharides have been shown to enhance the secretion of cytotoxic cytokines from human macrophage and the growth of human endothelial cells [7, 8]. They also possess other beneficial physiological activities [9, 10]. Alginate lyase has a potential medicinal application for enhancing antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms [11]. Recently, algae have attracted much attention for the production of biofuel such as biodiesel and bioethanol because algae can convert carbon dioxide to biomass by using sunlight [12]. Alginate has a potential as a substrate for the production of bioenergy [13, 14]. When alginate is used as the renewable source for biofuel, the saccharification of alginate is a prerequisite, and alginate lyase can play a key role in the saccharification [15].
In biocatalytic process for the production of value-added bioproducts, re-use of enzymes by immobilization is often to the key to enhance the economic feasibility. Various immobilizations have been developed with some advantages and drawbacks for enzyme recycling. Recently, we have developed NiO nanoparticles assembled on the surface of magnetic silica [16, 17]. The superparamagnetic iron oxide and hybrid nanoparticle with a Fe3O4–silica–NiO superstructure possess the binding affinity to His-tagged proteins and magnetic controllability on the basis of flocculation and dispersion properties of Fe3O4 nanoparticles. The recombinant epoxide hydrolase has been tested as a model enzyme and efficiently anchored to the nanoparticles [18]. The resulting immobilized enzyme could be efficiently re-used.
Recently, we have cloned a novel alginate lyase from a marine microorganism, Streptomyces sp. ALG-5, by using PCR with the primer obtained from the homolog sequences of poly-guluronate lyases [2]. The ALG-5 alginate lyase exhibited a higher alginate-degradation activity for poly-G block than it did for poly-M block. In this paper, the ALG-5 alginate lyase was immobilized onto two types of the magnetic nanoparticles for preparing alginate oligosaccharides (Fig. 1). The alginate lyase gene was functionally expressed in various expression vectors and chaperone plasmids to obtain highly active marine alginate lyase. The immobilized enzyme was successfully re-used by magnetic separation in a repeated batch operation.
Materials and methods
Cloning and functional expression of ALG-5 alginate lyase gene
For the cloning of the ALG-5 alginate lyase gene without signal peptide in pColdI expression vector, the forward primer with EcoRI (5′-GAATTCGCCGCCCCGTGCGACTACCC-3′) and reverse primer with HindIII (5′ AAGCTTCAGGAGTGTGTGACCTGCAGCTTG-3′) were used. For pET-21b(+) expression vector, the forward primer with NdeI (5′-GCATATGGCCGCCCCGTGCGACTACCC-3′) and reverse primer with XhoI (5′-GCTCGAGGGAGTGTGTGACCTGCAGCTTG-3′) were used. The pColdI/ALG-5 or pET-21b(+)/ALG-5 recombinant plasmid was expressed in Escherichia coli BL21 (DE3) at 37 and 15 °C to investigate the effect of expression temperature.
The recombinant plasmids were also expressed in various E. coli BL21 harboring various chaperone genes (pG-KJE8, pGro7, pKJE7, pG-Tf2, and pTf16) (TaKaRa, Japan). The recombinant E. coli was cultured on a LB medium supplemented with 50 μg/ml ampicillin and 34 μg chloramphenicol/ml for 2 h up to optical density (600 nm) of 0.5 at 180 rpm and 37 °C. l-arabinose and tetracycline were added to final concentrations of 0.5 mg/ml and 5 ng/ml for the induction of chaperone expression. The recombinant cells were incubated at 15 °C for 24 h to express the ALG-5 alginate lyase gene by addition of 1 mM IPTG.
Purification of alginate lyase
The cells were harvested and sonicated in 100 mM potassium phosphate buffer (pH 7.2) by using ultrasonicator Vibra Cell VCX400 (Sonics & Materials Inc, USA). The cell homogenate containing His-tagged alginate lyase was loaded on a Ni-Sepharose column (Amersham Biosciences, USA) equilibrated with 100 mM phosphate buffer (pH 7.2) and 0.5 M NaCl. The column was washed with the same buffer containing 10 mM imidazole, and alginate lyase protein was eluted with the same buffer containing 500 mM imidazole. The active fraction was desalted using a HiTrap™ desalting column (Amersham Biosciences, USA).
Analysis of the expressed ALG-5 alginate lyase proteins
The cells were harvested by centrifugation, and re-suspended in 50 mM sodium phosphate lysis buffer containing 300 mM NaCl, 10 mM imidazole, 10% (v/v) glycerol, and 0.5% (v/v) Triton X-100. Lysozyme and phenylmethanesulfonyl fluoride were added to the final concentrations of 1 mg/ml and 1 mM, respectively. The suspension solution was homogenized by the ultrasonicator for 20 min with ice cooling. Then the lysate was incubated at 37 °C for 10 min. The cell debris was precipitated by centrifugation (13,000 rpm, 20 min) at 4 °C, and the supernatant was filtered by a membrane with a cut-off 0.2 μm to obtain a clear extract. The precipitate was re-suspended in the lysis buffer to get insoluble proteins after washing the cell debris with the same buffer. To analyze the effect of culture conditions on the expression of soluble proteins, the lysate, soluble and insoluble protein fractions were prepared at 95 °C for 10 min with Laemmli buffer. The proteins were separated on 12% (v/v) SDS-polyacrylamide gel, and then transferred onto a nitrocellulose membrane. The membrane was incubated with polyclonal antibody against hexahistidine (H-15, Santa Cruz Biotechnology Inc.) and peroxidase-conjugated anti-rabbit IgG (Jackson Immunoresearch), and then visualized with CN/DAB (4-chloronaphthol/3,3′-diaminobenxidine) Substrate Kit (Pierce, USA).
Assay of alginate lyase activity
Enzymatic degradation of alginate was done in 20 mM phosphate buffer containing 0.4% (w/v) sodium alginate at pH 7.0 and 37 °C. The formation of alginate oligosaccharides was analyzed by measuring the absorbance at 235 nm or by thiobarbituric acid (TBA) assay at 548 nm [19]. One unit of enzyme activity was defined as an increase of 1.0 in absorbance per minute. The reaction mixture was treated with absolute ethanol (ratio of reaction mixture to ethanol, 3:7). The reaction products were analyzed by TLC with a solvent ratio of 1-butanol to formic acid to water (4:6:1, v/v/v) and compared with the authentic mixture of dimer and trimer prepared by BioGel P2 gel-filtration chromatography (2.5 × 240 cm, Bio-Rad, USA) [2]. The depolymerized products were visualized by spraying 10% (v/v) sulfuric acid in ethanol.
In order to analyze the effects of pH and temperature on alginate lyase activity, enzymatic degradation reactions were carried out at various pH and temperature ranges. The temperature was changed from 20 to 80 °C. Four types of 10 mM buffers: acetate buffer (pH 3.6–5.8), potassium phosphate buffer (pH 5.6–8.0), Tris–HCl buffer (pH 7.0–10.0), and sodium carbonate buffer (pH 9.0–11.0) were used for the analysis of pH effect on alginate degradation by alginate lyase.
Preparation of the magnetic nanoparticles
Two types of magnetic nanoparticles were used in this study. One magnetic nanoparticle was the Ni-anchored magnetic nanoparticle (Ni-MNP) [17]. The superparamagnetic Fe3O4 nanoparticles were stabilized by oleic acid and coated with Pluronic copolymer by mixing with P123 in CHCl3 solution. The Pluronic copolymer coated magnetic nanoparticles (PCMNPs) were reacted with NiNO3 and NaBH4 for anchoring Ni2+ on the surface. The other magnetic nanoparticle, the PEG-modified hybrid Fe3O4/silica/NiO nanoparticle (PEG-HNP), was prepared based on the previous study [18]. The synthetic procedure was as follows: Fe3O4 nanoparticles with core diameter of 12 nm were synthesized, and then dispersed in cyclohexane. Tetraethylorthosilicate was added, and the solution was stirred for the synthesis of silica nanospheres containing Fe3O4 nanocores. Fe3O4 nanocores were functionalized with amine groups, and then mixed with NiO nanoparticles in a tetrahydrofuran/CHCl3 for the assembly of NiO nanoparticles. The resulting hybrid nanoparticles of Fe3O4/silica/NiO (HNP) superstructure were modified with PEG with 2-[methoxy(polyethylenoxy)propyl]trimethoxysilane (MPEOPS).
Immobilization of alginate lyase and enzyme recycling in a repeated batch operation
A 100 μg of the recombinant alginate lyase was added to 1 ml of 20 mM phosphate buffer containing 10 mg Ni-MNPs or PEG-HNPs, and the mixture was incubated for 30 min at 25 °C with mild shaking. After removing unbound proteins, the immobilized enzyme was used for the preparation of alginate oligosaccharides. The retention of the enzymatic activity after immobilization was determined by comparing the initial rates of the immobilized enzymes with that of free enzyme.
Re-use of the immobilized enzyme was carried out in a repeated batch mode. After 15 min reaction, the immobilized enzyme was recovered by a magnetic bar or centrifugation. For consecutive use, a new reaction buffer with 0.4% (w/v) alginate was added to the immobilized enzymes. The amount of the alginate oligosaccharides was determined by TBA assay.
Chemicals
All chemicals for the synthesis of magnetic nanoparticles were used as purchased without any purification. Sodium alginate (3,500 cps) was purchased from Sigma Co (USA).
Results and discussions
Functional expression and purification of the recombinant alginate lyase of Streptomyces sp. ALG-5
In order to develop an immobilized biocatalyst for the preparation of alginate oligosaccharides, the marine alginate lyase gene from Streptomyces sp. ALG-5 was heterologously expressed in E. coli BL21 with various expression vector and chaperones to obtain an active ALG-5 alginate lyase. First, the effect of expression temperature was investigated to enhance the activity of ALG-5 alginate lyase. The alginate lyase gene was expressed in pET expression vector at moderate temperature, 37 °C, and low temperature, 15 °C, respectively. The alginate-degradation activity of the same amount of the lysate expressed at 37 °C was about four times lower than that of low temperature because most of the gene products were produced as insoluble inclusion bodies when alginate lyase gene was expressed at 37 °C. On the contrary, the amount of the soluble form of the recombinant alginate lyase was enhanced at 15 °C, compared to that at 37 °C (Fig. 2). This result indicated that low temperature had a positive effect on the soluble expression of marine alginate lyase in E. coli. Low temperature can generally slow down translation rate and thus enhance the proper folding as a soluble form [20].
Analysis of the expressed alginate lyase in E. coli BL21 (DE3) with various expression vectors. a SDS-PAGE, b immunoblotting. M indicates standard marker. Lanes 1, 4, and 7 represent lysates. Lanes 2, 5, and 8 represent soluble proteins. Lanes 3, 6, and 9 represent insoluble proteins. Lanes 1, 2, and 3: pColdI/ALG-5 cultured at 15 °C; lanes 4, 5, and 6: pET-21b(+)/ALG-5 cultured at 37 °C; lanes 7, 8, and 9: pET-21b(+)/ALG-5 cultured at 15 °C
Based on the result that the marine alginate lyase gene could be more efficiently expressed at low temperature, we employed a low temperature expression vector, pColdI. The marine alginate lyase gene was expressed in pET or pColdI at 15 °C. As shown in Fig. 2, the alginate lyase gene in pColdI vector was successfully expressed in E. coli BL21 with a molecular mass of approximately 27.5 kDa. The expression level of alginate lyase in pColdI vector was higher than that in pET vector. Thus, we employed pColdI as the expression vector for the following experiments, even though the relative amount of insoluble form was still higher in the case of pColdI expression system at 15 °C.
Misfolding and insoluble aggregation of protein are major problems to decrease catalytic activity of recombinant enzyme. Molecular chaperones can play key roles in solving this problem [21]. Yet, although co-expression of molecular chaperone is expected to give positive effect on heterologous expression in E. coli, an optimization of co-expression should be determined by experiments. Therefore, we investigated the effect of co-expression of alginate lyase gene in pColdI vector in the presence of various chaperone genes. The expression level of alginate lyase with pColdI was the highest in the absence of chaperones since the expression level of alginate lyase was influenced by co-expression level of various chaperones. In order to compare the alginate-degrading activities of alginate lyase expressed in the presence or absence of chaperones, the relative initial degradation rates were compared each other. The alginate lyase in pColdI without chaperones showed the highest activity due to the high content of alginate lyase in the lysate (Fig. 3). In the case of co-expression of alginate lyase gene in pColdI and pG-KJE8 chaperone plasmid exhibited a relatively high activity even though the expression level was low. The alginate-degrading activity was half of that of pColdI in the absence of chaperones, while the expression level was below half, which indicates that the specific activity of the alginate lyase co-expressed with pG-KJE8 might be high. The pG-KJE8 plasmid contained the genes of dnaK-dnaJ-grpE and groES-groEL. The increase in the specific activity is most probably due to the increase in the relative population of active enzyme preparation in the presence of chaperones. However, when the alginate lyase was isolated from the recombinant cells with pG-KJE8 plasmid, the specific alginate-degrading activities were similar with that of pColdI in the absence of chaperones because the alginate lyase might lose the help of chaperones for its correct folding during the purification procedure. Based on the above experimental results, we selected the expression of alginate lyase gene in pColdI at low temperature as the method of preparing enzyme in the following experiments.
Comparison of degradation activities of cell lysates containing the recombinant alginate lyases expressed in the presence of various chaperones. The cell lysates contained 100 μg/ml proteins, and 0.4% (w/v) alginate was used as the substrate. The degradation reactions were conducted at 37 °C for 2 h
Effects of pH, temperature, and divalent metal ions on the activity of recombinant alginate lyase
In order to develop a biotransformation process for the preparation of alginate oligosaccharides, the effects of pH and temperature on alginate degradation by the purified recombinant alginate lyase was analyzed and optimized. Figure 4 shows the effect of temperature and pH on the degradation of alginate by the recombinant alginate lyase. The pH dependence was studied in the range pH 3.6–11.0. The alginate lyase of Streptomyces sp. ALG-5 appeared to be active at a pH-optimum range between 7.0 and 8.0 (Fig. 4a). The temperature was varied in the range between 20 and 80 °C, and the alginate lyase showed a maximum activity at 50 °C for initial rate experiments for 10 min reaction (Fig. 4b). Enzyme stability was relatively well maintained at 40 °C for 30 min reaction, and then sharply decreased with further increase in temperature (Fig. 4c). The optimal reaction temperature was determined to be 40 °C based on enzyme stability because the immobilized alginate lyase would be used for many times by recycling. We also investigated the effect of addition of divalent metal ions on alginate-degrading activity of the recombinant alginate lyase based on the result that divalent metal ions could enhance the alginate-degradation activity in some alginate lyases [1]. The activity of alginate lyase of Streptomyces sp. ALG-5 increased up to 70% in the presence of 1 mM Co2+, Mn2+, Mg2+ and Ca2+ (Fig. 5).
Nano-immobilization of the recombinant alginate lyase of Streptomyces sp. ALG-5 using the Ni-MNPs or PEG-HNPs
Enzyme immobilization can offer benefits of easy re-use of the enzyme. To date, there is no report on the immobilization of alginate lyase for the preparation of alginate oligosaccharides. We employed the Ni-MNPs and PEG-HNPs to evaluate the feasibility of the magnetic nanoparticles as immobilization media for the recombinant alginate lyase. The recombinant alginate lyase was expressed in pColdI vector at 15 °C, and then separated by using Ni-Sepharose columns. The purified alginate lyase with His-tag was immobilized onto the Ni-MNPs or PEG-HNPs displaying Ni2+ ions on their surfaces in 20 mM potassium phosphate buffer (pH 7.5). In order to determine the optimum enzyme loading, different amounts of the alginate lyase enzyme up to 200 μg/ml were mixed with 10 mg nanoparticles, and then gently shaken at 37 °C for 20 min. The activities and amount of protein of the supernatants were assayed, and the enzyme loadings were determined to be in the range from 10.0 to 15.0 μg/mg nanoparticles. In the following experiments, the enzyme loading was maintained at about 12.5 μg/mg nanoparticles.
After immobilization, the activity of the immobilized enzyme was compared with that of free enzyme, and the retentions of original activity were determined to be about 90% for both of Ni-MNPs and PEG-HNPs. High retention of enzyme activity after immobilization indicates the advantages of our immobilization system. The pH and temperature dependence of the immobilized alginate lyases were similar to that of free enzyme, which implies the immobilizations did not affect the catalytic nature of the alginate lyase.
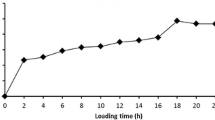
The immobilized alginate lyases were used for the degradation of 0.4% (w/v) alginate. The productions of alginate oligosaccharides were monitored by the TBA method measuring the released oligomers with unsaturated sugar at its reducing end. Re-uses of the immobilized alginate lyases were investigated by conducting repeated batch runs (Table 1). The relative amounts of alginate degraded by the recycled alginate lyase were measured for each round of recycling for the given reaction time. The immobilized enzymes were recycled by magnetic attraction. Both of the immobilized alginate lyases onto Ni-MNPs and PEG-HNPs were efficiently recycled more than five times with retention of about 85% of the initial activities. The performance of the PEG-HNPs-immobilized alginate lyase was better than that of Ni-MNPs. After the fifth cycle, the immobilized enzyme activities were decreased and the reaction times to be required to obtain same amount of alginate oligosaccharides were increased. The decrease in activity after 10 recycles was most probably due to the progressive deactivation of the enzyme itself because loss of the enzyme after each recycle was below 10%.
Figure 6 represents a production pattern of alginate oligosaccharide from alginate by the PEG-HNPs-immobilized alginate lyase. After the degradation of alginate by the immobilized alginate lyase, the reaction products were pretreated with ethanol that precipitates the alginate substrate and the higher molecular size oligosaccharides followed by TLC analysis. Free and immobilized enzymes exhibited similar patterns of alginate oligosaccharides production. As the degradation of 0.4% (w/v) alginate by the immobilized alginate lyase progressed, the total amount of alginate oligosaccharides increased. The relative concentrations of the different oligomers were changed with degradation time. The relative concentrations of dimer and trimer increased during the degradation of alginate, because the intermediates with high molecular weight were continuously degraded by the enzyme during the reaction. The reaction products were compared with the sample mixture of dimer and trimer determined by electrospray mass spectroscopy [2]. In conclusion, the re-use of the magnetic nanoparticle-immobilized alginate lyases for consecutive batch reactions could be used for the preparation of alginate oligosaccharides.
Time course of the TLC profile for monitoring the production of alginate oligosaccharides by the PEG-HNPs-immobilized alginate lyase. Each reaction mixture was lyophilized, spotted on silica G60 TLC plate and developed with 1-butanol:formic acid:water (4:6:1). Each spot represents the reaction products at time 1.5, 4, 8, 15, 30, 60 and 120 min, respectively. The two bands from top to bottom represent dimer and trimer, respectively
Conclusions
The Streptomyces sp. ALG-5 alginate lyase exhibiting polyG block-preferred alginate-degrading activity was functionally expressed, isolated, and immobilized onto the Ni-MNPs and PEG-HNPs. The immobilized enzymes were efficiently re-used in the repeated batch operation. Alginate oligosaccharides consisting of dimer and trimer were successfully prepared by recycling the immobilized alginate lyase more than 10 times with an excellent operational stability of more than 85%. The development of the immobilized alginate-lyase-catalyzed preparation of alginate oligosaccharides will facilitate the practical application of alginate oligosaccharides as a bioactive material.
References
Wong TY, Preston LA, Schiller NL (2000) Alginate lyase: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and application. Annu Rev Microbiol 54:289–340
Kim DE, Lee EY, Kim HS (2009) Cloning and characterization of alginate lyase from a marine bacterium Streptomyces sp. ALG-5. Mar Biotechnol 11:10–16
Zhang Z, Yu G, Guan H, Zhao X, Du Y, Jiang X (2004) Preparation and structure elucidation of alginate oligosaccharides degraded by alginate lyase from Vibrio sp. 510. Carbohydr Res 258:187–197
Kawamoto H, Horibe A, Miki Y, Kimura T, Tanaka K, Nakagawa T, Kawamukai M, Matsuda H (2006) Cloning and sequencing analysis of alginate lyase genes from the marine bacterium Vibrio sp. O2. Mar Biotechnol 8:481–490
Matsubara Y, Kawada R, Iwasaki K, Kimura Y, Oda T, Muramatsu T (2000) Cloning and sequence analysis of a gene (aly PG) encoding poly(α-l-guluronate) lyase from Corynebacterium sp. strain ALY-1. J Biosci Bioeng 89:199–202
Osawa T, Matsubara Y, Muramatsu T, Kimura M, Kakuta Y (2005) Crystal structure of the alginate (poly-α-l-guluronate) lyase from Corynebacterium sp. at 1.2 Å resolution. J Mol Biol 345:1111–1118
Iwamoto M, Kurachi M, Nakashima T, Kim D, Yamaguch K, Oda T, Iwamoto Y, Muramatsu T (2005) Structure–activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett 579:4423–4429
Kawada A, Hiura N, Tajima S, Takahara H (1999) Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch Dermatol Res 291:542–547
Cao L, Xie L, Xue X, Tan H, Liu Y, Zhou S (2007) Purification and characterization of alginate lyase from Streptomyces species strain A5 isolated from Banana Rhizosphere. J Agric Food Chem 55:5113–5117
Gimmestad M, Ertesvåg H, Heggeset TMB, Aarstad O, Svanem BIG, Valla S (2009) Characterization of three new Azotobacter vinelandii alginate lyases, one of which is involved in cyst germination. J Bacteriol 191:4845–4853
Alkawash MA, Soothill JS, Schiller NL (2006) Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS 114:131–138
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Beer LL, Boyd ES, Peters JW, Posewitz MC (2009) Engineering algae for biohydrogen and biofuel production. Curr Opin Biotechnol 20:264–271
Vasudevan PT, Briggs M (2008) Biodiesel production-current state of the art and challenges. J Ind Microbiol Biotechnol 35:421–430
Choi D, Ryu B-Y, Piao YL, Choi S-K, Jo B-W, Shin W-S, Cho H (2008) Studies on saccharification from alginate using Stenotrophomonas maltophilia. J Ind Eng Chem 14:182–186
Lee IS, Lee N, Park J, Kim BH, Yi YW, Kim T, Kim TK, Lee IH, Paik SR, Hyeon T (2006) Ni/NiO core/shell nanoparticles for selective binding and magnetic separation of histidine. J Am Chem Soc 128:10658–10659
Lee KS, Lee IS (2008) Decoration of superparamagnetic iron oxide nanoparticles with Ni2+: agent to bind and separate histidine-tagged proteins. Chem Commun 2008:709–711
Lee KS, Woo MH, Kim HS, Lee EY, Lee IS (2009) Synthesis of hybrid Fe3O4/silica/NiO superstructures and their application as magnetically separable high-performance biocatalysts. Chem Commun 25:3780–3782
Yoon H-J, Hashimoto W, Miyake O, Okamoto M, Mikami B, Murata K (2000) Overexpression in Escherichia coli, purification, and characterization of Sphingomonas sp. A1 alginate lyase. Protein Expr Purif 19:84–90
Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol 67:289–298
Schlieker C, Bukau B, Mogk A (2002) Prevention and reversion of protein aggregation by molecular chaperones in the E. coli cytosol: implications for their applicability in biotechnology. J Biotechnol 96:13–21
Acknowledgments
This work was supported by New & Renewable Energy R&D program (20093020090020) under the Korea Ministry of Knowledge Economy (MKE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, J.W., Choi, S.H., Kim, D.E. et al. Heterologous expression of an alginate lyase from Streptomyces sp. ALG-5 in Escherichia coli and its use for preparation of the magnetic nanoparticle-immobilized enzymes. Bioprocess Biosyst Eng 34, 113–119 (2011). https://doi.org/10.1007/s00449-010-0452-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0452-4